Observational, prospective, open and multi-center study to evaluate the efficacy and safety of Tendoactive® in patients with tendinopathies
Objective
To evaluate the efficacy and safety of Tendoactive® on the clinical and structural evolution of the tendinopathies affecting the
Achilles, patellar, and lateral epicondyle.
Methods
A multi-center, open-label, non-comparative, prospective, exploratory phase IV study was performed.
A total of 98 tendinopathy patients participated in this study (32 Achilles, 32 patellar, 34 lateral epicondyle). Each patient received a daily dose of Tendoactive®* over a period of 90 days.
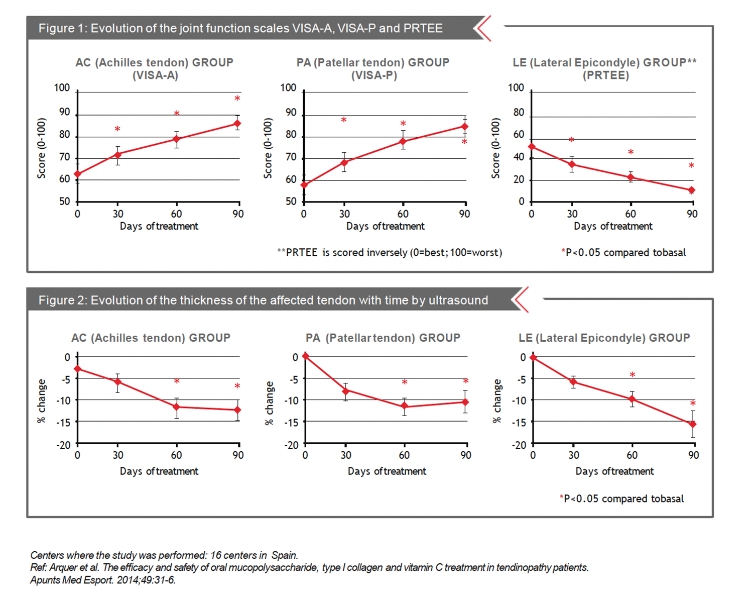
Every month, pain at rest and during activity of the affected tendon was assessed using a VAS scale. In addition, joint function was assessed using the VISA -A,VISA- P and PRTEE questionnaires. Finally the affected tendon was examined and characterized by ultrasound.
Results
A significant reduction in pain both at rest and during activity was observed between the first control visit (day30) and the end of the study (day90) for all three types of tendinopathies. Improvements of 38% in VISA-A, 46% in VISA- P and 77% in PRTEE were observed at the conclusion of the study on day 90 (P<0.001) (Figure1). Similarly, a 12 % decrease in the thickness of the Achilles tendon, a 10% decrease in the patellar tendon and a 20% decrease in the lateral epicondyle tendon was observed(P<0.05)(Figure2).
There was a reduction in analgesic consumption and no treatment-related adverse events were reported during the study.
* Tendoactive® contains mucopolysaccharides, collagen type I and vitaminC