Observational and prospective study to evaluate the efficacy of Tendoactive® in the treatment of tendinopathies
Objective
To evaluate the effectiveness of Tendoactive® administration in the treatment of different tendinopathies, such as epicondylitis, supraspinous tendinopathy, Achilles tendinopathy and plantar fasciitis.
Methods
20 patients per clinical condition were recruited for this study. Their conditions were clinically and ecographically verified, resulting in a total of 80 study participants.
Patients were randomly assigned to either a treatment group (n=10) or a control group (n=10). Over a period of three months, all the patients received between 20 and 30rehabilitation sessions. In addition, the study group consumed Tendoactive® (2.16g/day).
Patient progress was evaluated using the SF 36 Quality of Life Survey, Standardized Functional Assessment, and VAS scale for pain levels. Patients completed all evaluations prior to study commencement to establish baseline and then one, two- and three-months during treatment.
Results
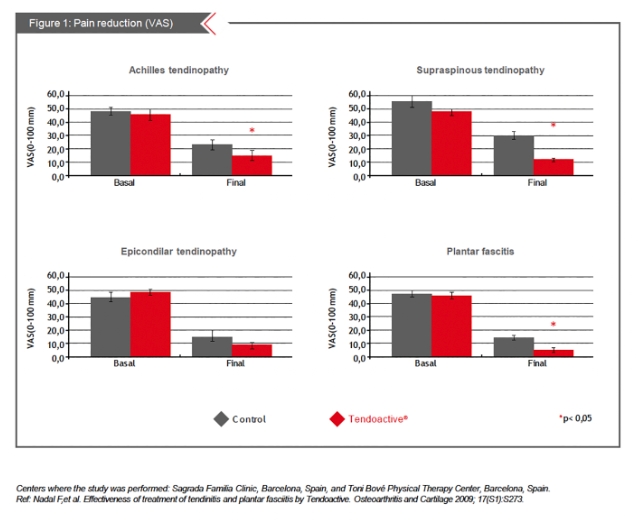
All subgroups that received Tendoactive® treatment displayed a significant reduction in pain (P<0.05) with the exception of those with epicondylitis (Figure1). Pain reduction was associated with an improvement of at least one SF- 36 subscale in each subgroup (P<0.05). At the end of the treatment a significant improvement in the functional assessment of the physiotherapist was reported for all tendinopathies (P<0.05).
Conclusion
The results suggest that the use of Tendoactive® for the management of tendinopathies and plantar fasciitis is safe and effective, leading to a significant reduction in pain and improving the properties of the tendon, without adverse effects.